easyClinical Supports Innovative MedTech Solutions
We help MedTech companies in both clinical evaluation and clinical investigation through generating robust clinical evidence to get their products authorized on the market and advancing patient care and medical innovation.
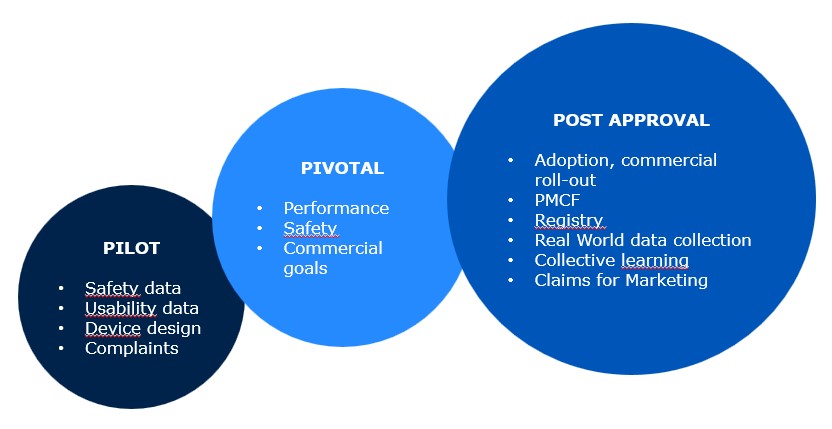
easyClinical supports innovators with all requirements for all risk classes of their device-Class, including Post market clinical follow-up (PMCF).
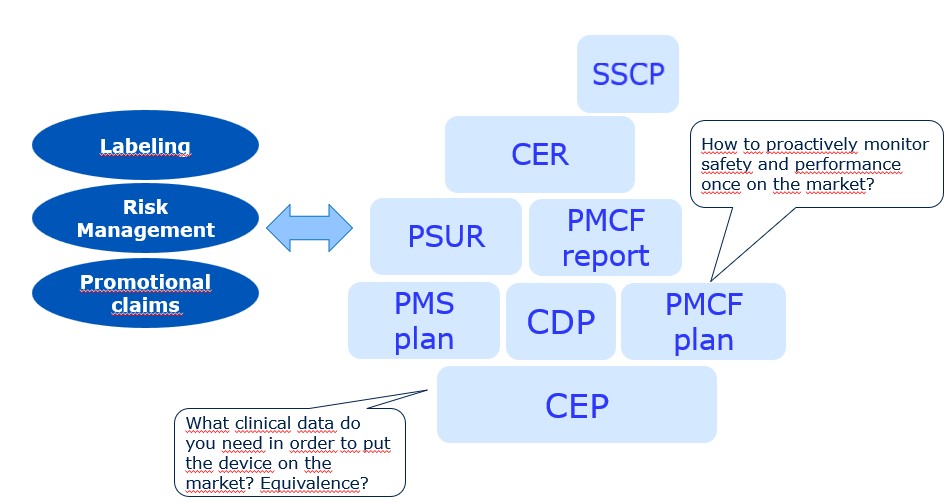
Clinical Evaluation
- Clinical Evaluation Report (CER)
- Clinical Evaluation Plan (CEP)
- Clinical Development Plan (CDP)
- PMCF Plan
- Post-Market Surveillance Plan (PMS)
- Periodic Safety Update Report (PSUR)
- Summary of Safety and Clinical Performance (SSCP)
- Claiming Equivalence
- Labeling
- Device Risk Management
Clinical Investigation
- Pre-market study
- Post-Market Clinical FU (PMCF) study
- Real-World data collection (retro prospective)
- PMCF Surveys
Capabilities
A business strategy is the means by which it sets out to achieve its desired ends.
If you have been selected for a business audit, here is what you need to know.
Restructuring your company could restore its viability and improve its liquidity position.
Benefits
Research helps you plan the best way to get your product from the manufacturer to the retail shelf. In addition to deciding which retailers should carry your product, you should determine where your inventory will be held.
Go to your team, and tell them you want to make a fresh start. Tell them you want them to enjoy their jobs more and get more done.
In this problem solving step, you will want to figure out what caused the problem, what the problem looks like at this moment, and the urgency of addressing the problem.
Insights
- Titre de l’article de blog July 30, 2024
- US Stocks Are Expensive April 11, 2017
- Investment Update, Fourth Quarter 2016 September 1, 2014
- Four Big Mistakes Your Small Business Is Making March 22, 2014
- 9 Resources to Get You Prepared for Tax Season March 21, 2014