Passionate about supporting clinical sites
Our focus is to protect the safety of study patients, the validity and integrity of study results while keeping the study running according to protocol and applicable regulatory requirements.
Our team of monitors is experienced with medical or scientific background working for several years on different therapeutic areas. Our global network of monitors allow us to perform monitoring visits in many countries.
Our approach is flexible and depends on protocol and study phase needs. Our partners could develop a systematic or risk-based monitoring approach. Also, they may choose on-site or remote monitoring, or a combination of on-site and remote monitoring.
Capabilities
A business strategy is the means by which it sets out to achieve its desired ends.
If you have been selected for a business audit, here is what you need to know.
Restructuring your company could restore its viability and improve its liquidity position.
Benefits
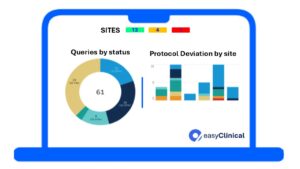
At easyClinical we leverage data management systems to ensure that patient data is collected in real-time through eCRF, reducing the risk of data entry errors.
We encourage continuous monitoring to identify discrepancies, missing data, or protocol deviations early on.
We use automated data verification processes to ensure that the information is accurate and reliable before it's used for decision-making.
We provide our partners with predictive analytics tools and study metrics to optimize patient enrollment, detect issues in patient data that might indicate adverse events, thus enabling quicker responses, ultimately leading to better decision-making and faster trial completion.
Effective data monitoring leads to short trial timelines and low study costs. At easyClinical we ensure that for each monitoring visit done, the likelihood of study outcomes release is increased.
Insights
- Titre de l’article de blog July 30, 2024
- US Stocks Are Expensive April 11, 2017
- Investment Update, Fourth Quarter 2016 September 1, 2014
- Four Big Mistakes Your Small Business Is Making March 22, 2014
- 9 Resources to Get You Prepared for Tax Season March 21, 2014